News & Insights
We offer valuable resources regularly, including compliance updates, new methods, expanded coverage, and technical how-tos to help businesses navigate secure digital transactions.
.png)
eID Easy Monthly Update: February 2026
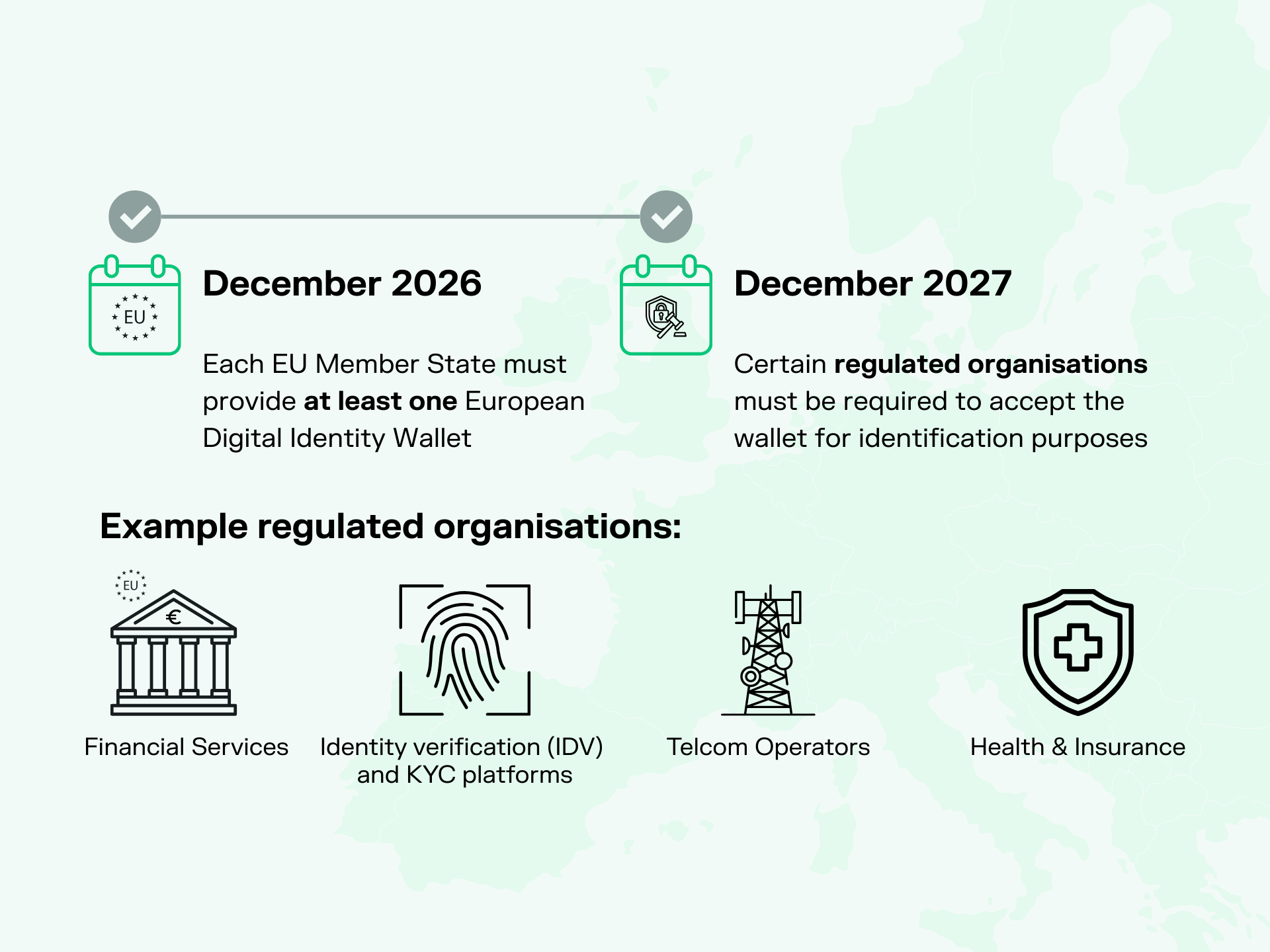
Less Than Two Years to Go: Is Your Organisation Expected to Support the EU Digital Identity Wallet in 2027?
Latest articles
.png)
eID Easy Monthly Update: February 2026
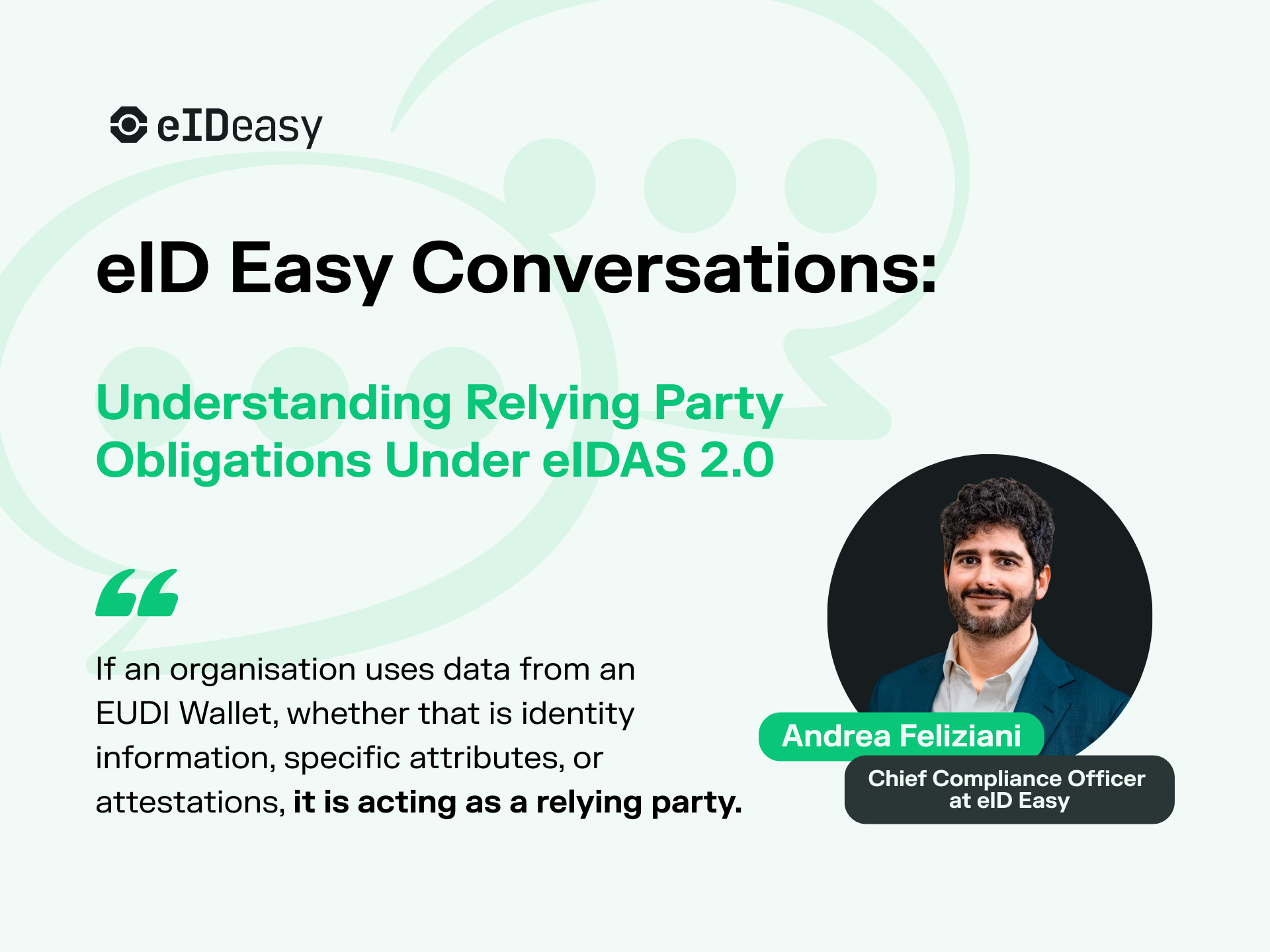
eID Easy Conversations: Understanding Relying Party Obligations Under eIDAS 2.0

EU Digital Identity Wallet (EUDIW): A Comprehensive FAQ for Businesses

Less Than Two Years to Go: Is Your Organisation Expected to Support the EU Digital Identity Wallet in 2027?

eID Easy Monthly Update: January 2026
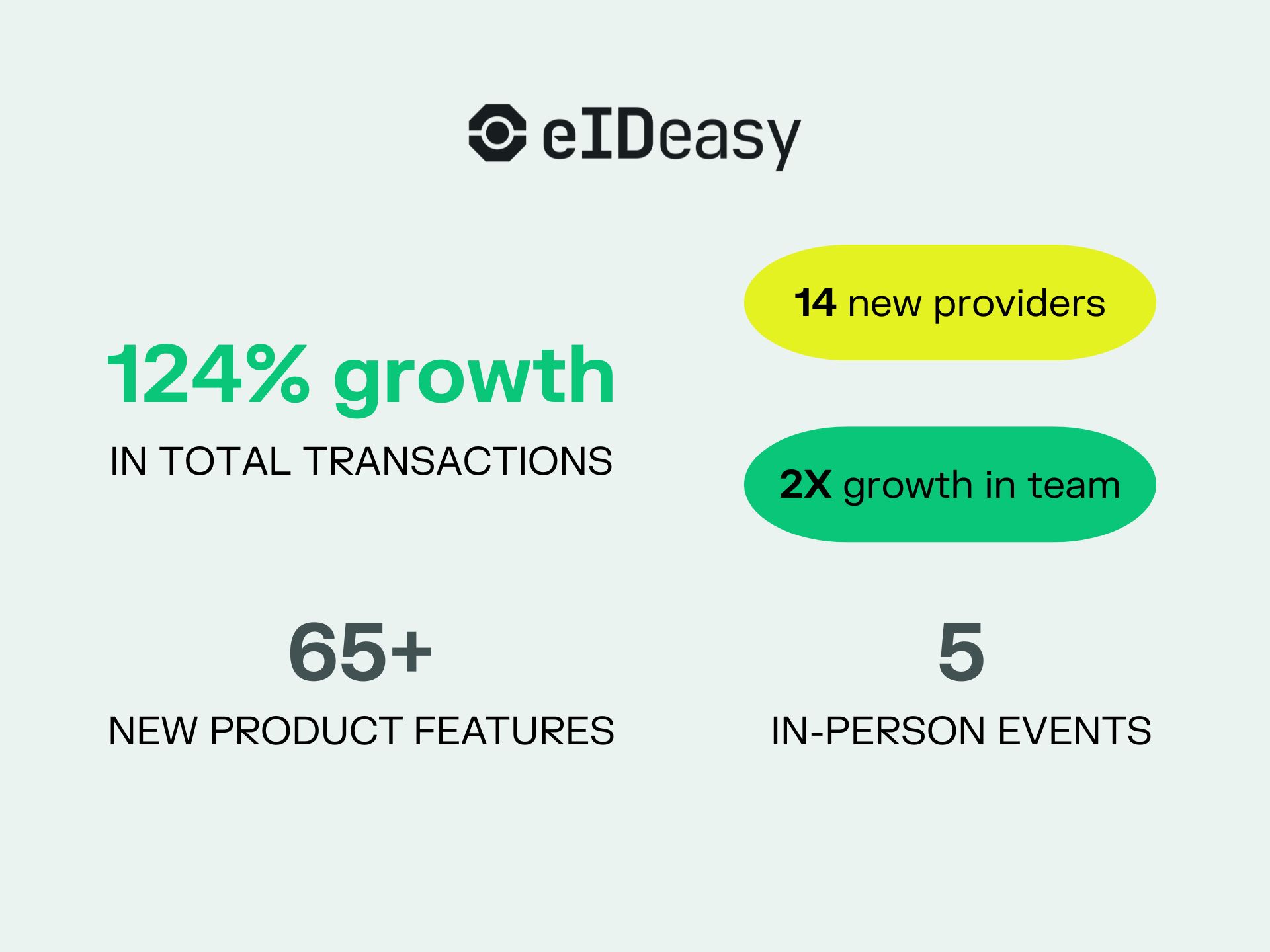
2025 Was Anything but Small: eID Easy’s Year in a Nutshell
Season’s Greetings from the Team: Our Holiday Card Signed by 18 People Across 13 Identity Providers

Choosing the Right Electronic Signature Type: A Practical Guide

eID Easy Product Update: November 2025

Goodbye Passwords: Key Takeaways from Authenticate 2025 and What’s Next for Wallets and eIDs
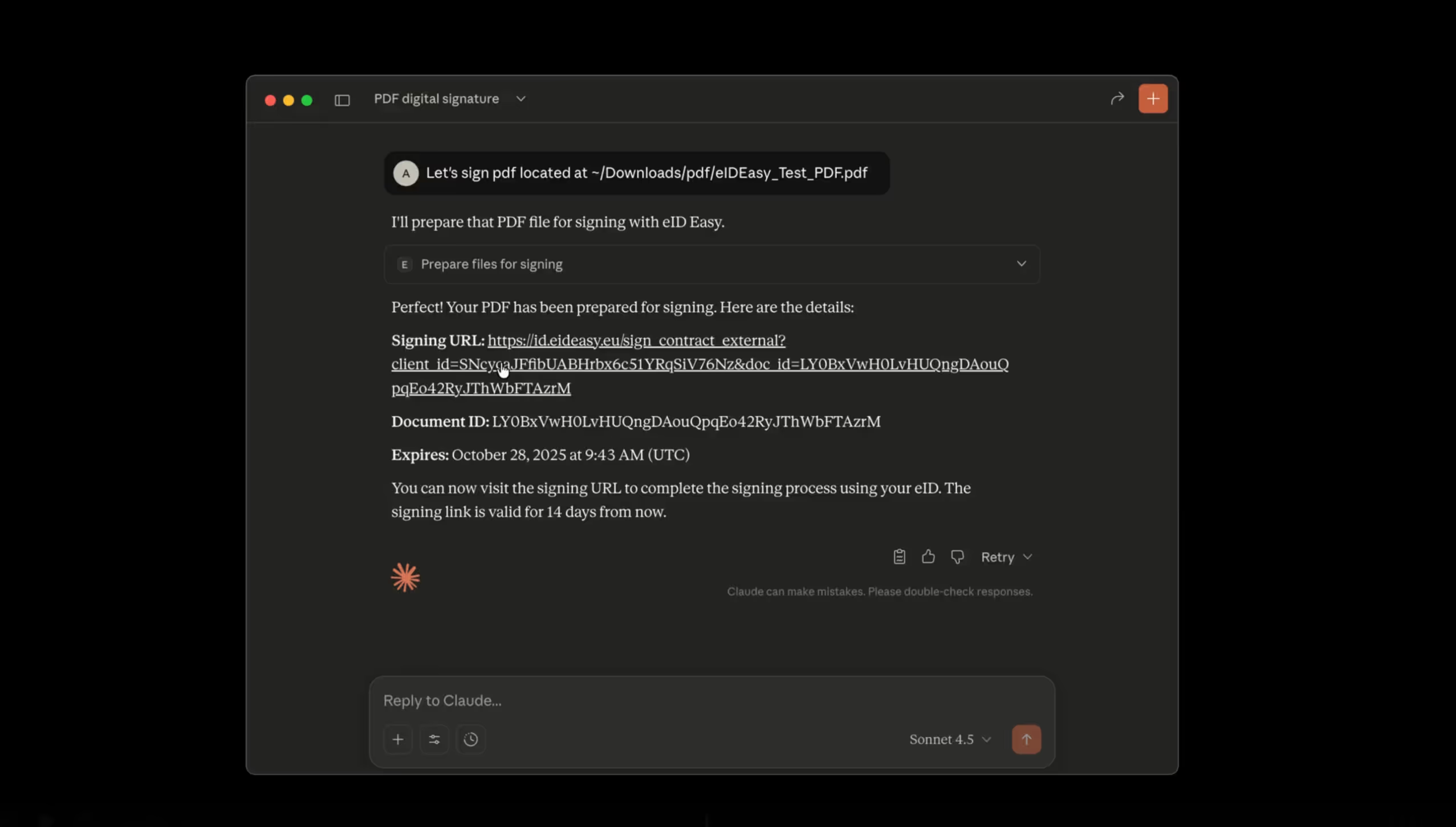
eID Easy MCP Server: Giving AI Agents the Power to Sign

